Introduction
Lithium-ion batteries (LIBs) power a vast range of modern devices, from smartphones to electric vehicles (EVs). They are also a crucial energy source for Personal Light Electric Vehicles (PLEVs) such as e-scooters and e-bikes, which are widely used in urban transportation. These batteries offer high energy density, long life cycles, and fast charging capabilities, making them the preferred choice for mobility applications.
However, thermal runaway (TR) remains one of the most significant safety concerns associated with lithium-ion batteries. When a battery cell overheats beyond a critical threshold, it enters an uncontrollable self-heating cycle, releasing large amounts of heat and toxic gases. If not contained, TR can lead to battery fires and explosions, causing severe property damage, injuries, and even fatalities.
With PLEV battery fires becoming increasingly common, there is an urgent need to understand, predict, and prevent thermal runaway events. This article provides an in-depth examination of what causes TR, how it spreads, and the most effective safety measures to mitigate risks.
What is Thermal Runaway?
Thermal runaway is a chemical chain reaction that occurs inside a lithium-ion battery when heat generation exceeds heat dissipation. The process is self-sustaining, meaning that once it begins, it accelerates uncontrollably until all the combustible material within the battery is consumed.

Key Characteristics of Thermal Runaway
Thermal runaway is a dangerous and self-sustaining reaction in lithium-ion batteries that occurs when heat generation exceeds the battery's ability to dissipate it. This uncontrolled process leads to extreme temperatures, gas emissions, and, in severe cases, fires or explosions. Once thermal runaway begins, it is nearly impossible to stop until the battery has fully decomposed. Below are the key characteristics of this phenomenon, explained in detail.
1. Uncontrollable Heat Build-up
One of the first warning signs of thermal runaway is a rapid temperature increase within the battery cell. Typically, lithium-ion batteries function safely within a temperature range of 0°C to 60°C, but when a cell reaches 150°C to 180°C, an exothermic (heat-releasing) reaction begins within the electrolyte and electrode materials. This reaction produces additional heat, which further raises the temperature of the battery.
Unlike overheating from external sources, which can be stopped by cooling, thermal runaway is self-perpetuating. The internal chemical reactions release more heat than the cell can dissipate, which further accelerates the reaction. As a result, the cell temperature can climb beyond 1000°C, making it impossible to halt the process once it has started. This extreme heat can also spread to neighboring cells, leading to a chain reaction of failures in a battery pack, known as thermal propagation.
2. Gas Venting and Cell Rupture

As the temperature inside the battery rises, the electrolyte—a flammable organic solvent—begins to break down into gases. These gases include hydrogen, carbon monoxide, methane, and other toxic byproducts, which rapidly build up pressure within the sealed cell casing. If this pressure becomes too high, the battery casing can rupture, releasing a burst of hot gases, flames, and possibly molten metal.
Before reaching full thermal runaway, some lithium-ion cells experience a venting phase, where smoke or gas leaks out. While this may not immediately cause combustion, the emitted gases are often highly flammable. If an ignition source is present—such as a spark, high heat, or another cell in thermal runaway—these gases can catch fire and cause an explosion.
Additionally, if the cell rupture happens in an enclosed space, such as an indoor environment, the accumulated gases can create an explosive vapor cloud. If ignited, this vapor cloud can result in a violent explosion, causing significant damage to property and posing a severe risk to human life.
3. Fire, Explosion, and Toxic Fumes
Once thermal runaway reaches its peak, the battery can combust violently, producing sustained flames that exceed 1000°C. At this temperature, even metal components within the battery can melt, leading to molten metal ejecta being forcefully expelled from the battery casing. These ejecta are highly dangerous, as they can ignite nearby flammable materials, furniture, or even adjacent battery cells, worsening the fire.
Unlike ordinary fires, lithium-ion battery fires are extremely difficult to extinguish. This is because, unlike traditional combustion, the fire does not rely on external oxygen. Instead, the battery itself supplies the necessary oxygen through the decomposition of its cathode material (such as Nickel-Manganese-Cobalt Oxide). This means that even if a fire extinguisher is used or oxygen is removed, the fire may continue to burn.
Firefighters often struggle to control lithium-ion battery fires, as they may require large amounts of water, foam, or specialized fire suppression agents to cool the cells. In cases where water is ineffective, burning batteries must be isolated and left to burn out completely.
In addition to fire, toxic fumes are another significant hazard associated with thermal runaway. When a battery undergoes this reaction, it emits dangerous chemicals, including hydrogen fluoride (HF), which is highly corrosive and can cause severe respiratory damage if inhaled. These toxic emissions pose a serious risk to firefighters, emergency responders, and anyone nearby when a lithium-ion battery fire occurs.
4. Thermal Propagation and Battery Pack Failures
When a single lithium-ion cell enters thermal runaway, it does not necessarily remain isolated. In battery packs, such as those used in e-scooters, e-bikes, and electric vehicles (EVs), multiple cells are packed closely together with minimal spacing. This design improves energy density but also increases the risk of thermal propagation—a chain reaction where one failing cell ignites neighboring cells.
Heat transfer can occur in multiple ways:
- Direct Contact – The high temperature from the failing cell conducts heat to adjacent cells.
- Gas and Flame Ejection – The vented gases and flames from the ruptured cell ignite nearby cells.
- Electrical Pathway Overload – A short circuit in one cell can send excessive current through the entire battery pack, triggering more failures.
- Structural Damage – The rupture of the battery pack casing can expose internal components to air, moisture, or sparks, further increasing the risk of ignition.
This cascading failure can quickly engulf an entire battery pack, leading to explosive fires in electric vehicles, energy storage systems, or consumer electronics. In some cases, entire buildings or vehicles have been destroyed due to the inability to contain or stop thermal propagation once it begins.
Causes of Thermal Runaway
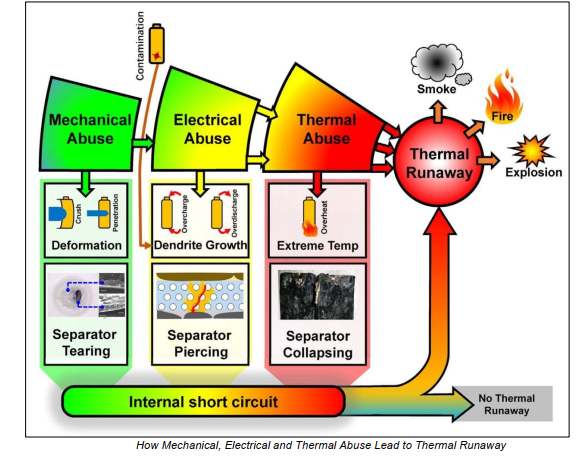
Thermal runaway in lithium-ion batteries occurs when heat generation surpasses the battery’s ability to dissipate heat, leading to an uncontrollable rise in temperature. This phenomenon can be triggered by a variety of factors, including mechanical damage, electrical stress, thermal exposure, and manufacturing defects. Each of these conditions can initiate the failure of a single battery cell, which may then lead to a chain reaction of failures across an entire battery pack. Below, we explore each of these causes in greater detail.

1. Mechanical Abuse
Mechanical abuse refers to physical damage that a lithium-ion battery may experience due to external forces such as crushing, puncturing, impact, or excessive vibration. The internal structure of a battery is highly sensitive, and even minor mechanical damage can create conditions that lead to thermal runaway.
Crushing and Puncturing: When a battery cell is crushed or punctured, it can cause a short circuit by forcing the anode and cathode into direct contact. The separator, which is a thin polymer layer designed to keep the electrodes apart, may be torn or compressed, leading to an electrical short. This uncontrolled electrical current generates rapid and intense heating, which can quickly trigger thermal runaway. In severe cases, the puncture itself can ignite flammable electrolytes, causing an immediate fire or explosion.
Impact Damage from Drops or Collisions: Many personal light electric vehicles (PLEVs), such as e-scooters and e-bikes, are frequently subjected to jolts, drops, and collisions. If a battery pack is dropped from a significant height or experiences a strong collision, it can suffer internal damage that may not be immediately visible. Over time, internal stresses can develop, causing internal shorts that eventually lead to thermal runaway.
Vibration and Structural Fatigue: E-bikes and e-scooters experience constant vibration while in use, especially on rough terrain. Prolonged exposure to vibrations can weaken battery welds, misalign cell layers, or cause tiny cracks to form inside the cell. These micro-damages may increase electrical resistance, leading to localized overheating and eventual failure of the battery cell.
2. Electrical Abuse
Electrical abuse occurs when a battery is overcharged, over-discharged, or subjected to excessive currents, all of which can lead to internal failures that trigger thermal runaway.
Overcharging: Lithium-ion batteries are designed to operate within a strict voltage range, typically around 3.0V to 4.2V per cell. If a battery is charged beyond this limit—often due to a faulty charger or inadequate Battery Management System (BMS)—it can cause lithium plating on the anode. This leads to the formation of lithium dendrites, which are tiny, needle-like structures that can grow through the separator and cause an internal short circuit. Once a short circuit occurs, heat production skyrockets, and thermal runaway becomes inevitable.
Over-Discharging: If a lithium-ion battery is discharged below its safe voltage threshold (typically 2.5V per cell), it can cause copper dissolution in the anode. This process weakens the cell structure and, upon recharging, can cause copper to reform as metallic deposits inside the battery. These deposits create unintended conductive pathways, leading to an internal short circuit when the battery is used again.
Short Circuits: Internal short circuits can be caused by manufacturing defects, damage to the separator, or contaminants inside the cell. External short circuits, on the other hand, can occur when wires are frayed, connectors are damaged, or battery terminals are bridged by a conductive object. A short circuit allows unrestricted current flow, generating intense heat within the battery and dramatically increasing the risk of thermal runaway.
Faulty Battery Management System (BMS): A well-designed BMS plays a crucial role in preventing electrical abuse by monitoring voltage, current, and temperature within the battery. However, a malfunctioning or low-quality BMS may fail to detect dangerous conditions, such as overcharging, overheating, or excessive discharge, allowing them to persist until the battery fails catastrophically.
3. Thermal Abuse
Thermal abuse refers to excessive heat exposure that can cause a battery to overheat beyond its safe operating limits, leading to thermal runaway. Unlike mechanical or electrical abuse, which may require direct interaction with the battery, thermal abuse can occur passively due to environmental conditions or poor system design.
Exposure to High Temperatures: Lithium-ion batteries are highly sensitive to temperature fluctuations and should typically be operated within a safe temperature range of 0°C to 60°C. If a battery is left in direct sunlight, a hot vehicle, or near a heat source, it can reach dangerously high temperatures. This external heating can accelerate internal chemical reactions, increasing internal pressure and gas buildup, eventually leading to cell rupture and ignition.
Poor Cooling and Ventilation: In battery packs, multiple cells are arranged tightly together. If the battery pack lacks proper ventilation or thermal management, heat generated by normal operation may become trapped. As temperatures rise, certain electrolyte decomposition reactions may begin at as low as 85°C, potentially triggering thermal runaway even without external heat sources.
Proximity to Other Heat Sources: If a battery is placed near other high-power electronic devices, heating elements, or electrical arcs, the ambient heat may be transferred into the battery cells. In some cases, neighboring cells that are already experiencing mild overheating can further accelerate the heating process, making thermal runaway more likely.
4. Manufacturing Defects and Contamination
Lithium-ion batteries are highly complex electrochemical devices, and their manufacturing process must be extremely precise to ensure safety and reliability. If contaminants or defects are introduced during manufacturing, they can significantly increase the risk of internal failures that lead to thermal runaway.
Metallic Contaminants: One of the most dangerous manufacturing defects is the presence of microscopic metal particles inside the battery. These tiny fragments, often left over from the production process, can penetrate the separator, causing a latent short circuit. Over time, this defect degrades the internal structure, eventually leading to an electrical failure and heat buildup that results in thermal runaway.
Defective Separators: The separator inside a lithium-ion battery is only about 15 microns thick and is responsible for keeping the anode and cathode apart. If a battery is produced with a weak or inconsistent separator, it may fail prematurely, allowing the electrodes to come into contact and trigger an internal short circuit.
Counterfeit or Low-Quality Cells: Many cheap, unbranded lithium-ion cells do not undergo rigorous quality control testing. Counterfeit batteries often lack safety features such as current interrupt devices (CIDs) and positive temperature coefficient (PTC) protections, making them far more susceptible to thermal runaway.
Poor Battery Assembly: Even if individual cells are manufactured correctly, improper assembly of a battery pack can create electrical imbalances, poor heat dissipation, or weak structural integrity. These factors increase stress on the cells, making them more vulnerable to thermal failure over time.
How Does Thermal Propagation Occur?
Thermal propagation is a chain reaction that occurs when thermal runaway spreads from one failing battery cell to others in a battery pack. In devices like e-scooters, e-bikes, and electric vehicles (EVs), lithium-ion batteries are typically composed of multiple cells packed closely together to maximize energy density. While this compact design enhances performance, it also increases the risk that a single failing cell can trigger a catastrophic battery fire.

Once thermal runaway initiates in a single cell, it can spread rapidly due to heat transfer, flame exposure, electrical imbalances, and structural damage. The key mechanisms that contribute to thermal propagation are explained in detail below.
1. Direct Contact Heat Transfer Between Cells
In most battery packs, cells are arranged tightly together to save space and improve energy efficiency. However, this design means that when one cell undergoes thermal runaway, the extreme heat it generates can directly transfer to adjacent cells, raising their temperature.
- Heat transfer occurs through physical conduction, meaning that if two cells are in direct contact, heat from the failing cell will quickly spread to its neighbors.
- If an adjacent cell reaches its own critical temperature (typically 150°C - 180°C), it will also enter thermal runaway, continuing the chain reaction.
- The higher the initial temperature of the failing cell, the faster the heat spreads to surrounding cells.
Battery manufacturers often add insulation or thermal barriers between cells to slow down heat transfer, but if these barriers are not thick enough or fail under extreme temperatures, they may be ineffective at preventing thermal propagation.
2. Ejected Flames and Hot Gases from a Failing Cell
When a lithium-ion cell reaches thermal runaway, it produces a violent expulsion of flames, gases, and molten metal ejecta. This material can directly impact neighboring cells, triggering their failure.
- The electrolyte inside the battery breaks down into flammable gases, such as hydrogen and methane, which ignite upon exposure to extreme heat.
- The forceful eruption of flames and gases from a venting cell can ignite surrounding materials and cause a fire to spread rapidly.
- If the battery pack does not have proper venting or gas release channels, the internal pressure buildup can explode the battery casing, accelerating propagation.
One of the biggest risks is that hot ejecta can physically puncture neighboring cells. This directly compromises their protective casings, exposing their electrolyte to heat and initiating immediate thermal runaway.
3. Electrical Pathway Overload
In a multi-cell battery pack, all the cells are electrically connected to provide the required voltage and current for operation. When one cell short circuits due to internal damage, it can create a dangerous current surge that affects the entire system.
- The failing cell draws excessive current from nearby cells, heating them up and stressing their internal components.
- If the battery does not have proper circuit protection (such as fuses or a Battery Management System, BMS), the sudden voltage drop may overload the entire battery pack.
- High electrical currents can melt battery contacts, leading to sparking and arcing, which can ignite flammable gases.
This form of thermal propagation is particularly dangerous because it can spread failure across an entire battery pack, even if some cells are not in direct physical contact with the failing cell.
4. Radiation Heating from the Failing Cell
Heat transfer in a battery pack does not only happen through direct contact. Even if some space exists between cells, thermal runaway can still spread via radiation heating.
- When a cell enters thermal runaway, it emits intense infrared radiation (heat energy).
- If the battery lacks thermal barriers, this radiated heat can increase the temperature of surrounding cells, bringing them closer to their own thermal failure threshold.
- Even if a nearby cell does not reach full thermal runaway, prolonged exposure to radiation heat can cause decomposition of its electrolyte, gas formation, and eventual rupture of the battery casing.
This form of thermal propagation is particularly common in battery packs with high energy density, where multiple high-capacity cells are closely arranged with minimal heat dissipation design.
5. Structural Damage Leading to Additional Failures
When thermal runaway occurs, it weakens the physical integrity of the entire battery pack. This can lead to mechanical failures that increase the likelihood of further thermal propagation.
- Battery casings, especially plastic enclosures, may melt or crack, exposing more cells to oxygen and external heat sources.
- If the battery pack is housed inside a vehicle or enclosure, its protective shell may warp or deform, compressing other cells and increasing their risk of mechanical failure.
- Structural damage can also disrupt cooling systems (if present), eliminating a key defense mechanism against further overheating.
In extreme cases, battery packs may rupture violently, causing large-scale fire events that make containment nearly impossible.
How to Prevent Thermal Runaway
Preventing thermal runaway in lithium-ion batteries requires a multi-layered approach that includes battery design improvements, enhanced management systems, safer materials, and better user practices. Since thermal runaway is often triggered by a combination of mechanical, electrical, and thermal stresses, effective prevention strategies must address each potential failure mode comprehensively.
Improved Battery Pack Design for Heat Management
One of the most effective ways to prevent thermal runaway is through better battery pack design, which minimizes the risk of excessive heat buildup and thermal propagation. Since lithium-ion cells are highly sensitive to temperature fluctuations, battery packs should be designed with proper spacing, thermal insulation, and heat dissipation mechanisms.
Many modern battery packs include thermal barriers between individual cells to slow down heat transfer. These barriers are often made of ceramic coatings, aerogels, or phase-change materials, which provide insulation while allowing controlled heat dissipation. Another design feature is passive cooling, where the battery pack is structured to allow natural air circulation around cells, preventing localized overheating.
For high-energy applications such as electric vehicles (EVs) and large-scale energy storage, manufacturers have incorporated active cooling systems that use liquid cooling or air-cooling technology. In automotive-grade lithium-ion batteries, liquid cooling loops help maintain stable cell temperatures even during high-power charging and discharging. Although active cooling is rare in e-scooters and e-bikes due to weight and space constraints, some high-end models include heat-dissipating materials or fan-assisted cooling systems to manage thermal loads effectively.
Battery Management Systems (BMS) for Early Fault Detection
A well-designed Battery Management System (BMS) is critical for monitoring and preventing conditions that could lead to thermal runaway. The BMS is an electronic control unit that continuously tracks the voltage, current, and temperature of each cell in the battery pack. If abnormal values are detected—such as overcharging, over-discharging, or unexpected heating—the BMS takes action to shut down charging, reduce current flow, or disconnect the faulty cell from the rest of the pack.
Advanced BMS software also implements cell balancing, which ensures that all cells in a battery pack maintain equal voltage levels. Without cell balancing, some cells may experience higher stress than others, making them more likely to overheat and trigger thermal runaway. Some high-end BMS systems also feature wireless monitoring and real-time alerts, enabling users to identify potential battery issues before they escalate into failures.
The most effective BMS designs not only detect temperature rise but also respond dynamically by modifying charging parameters or activating cooling mechanisms. However, not all PLEVs have a sophisticated BMS system, and low-cost batteries may include only minimal safety protections. Consumers should always ensure that their battery packs comply with international safety standards, such as UN 38.3, IEC 62133, and UL 2271, which require stringent testing for thermal stability and electrical safety.
Using Safer Battery Chemistries to Reduce Risk
Lithium-ion batteries come in several different chemistries, and some are inherently safer than others. Nickel-Manganese-Cobalt (NMC) and Nickel-Cobalt-Aluminum (NCA) batteries are commonly used in PLEVs because they offer high energy density and long battery life. However, these cobalt-based batteries are also more susceptible to thermal runaway, as they release significant heat and oxygen when they fail, making fires difficult to extinguish.
A safer alternative is Lithium Iron Phosphate (LFP) batteries, which have higher thermal stability and are less likely to undergo thermal runaway. Unlike NMC and NCA batteries, LFP cells do not release oxygen during decomposition, which reduces the likelihood of sustained combustion. While LFP batteries have lower energy density, they are gaining popularity in electric vehicles, stationary energy storage, and high-performance e-bikes due to their superior safety profile and longer cycle life.
As battery technology advances, solid-state batteries are emerging as a potential solution to eliminate thermal runaway entirely. Solid-state batteries replace liquid electrolytes with solid materials, which are non-flammable and highly resistant to overheating. Research into solid-state technology is ongoing, and it is expected that commercial adoption will significantly improve battery safety in the coming years.
Environmental Controls and Safe Charging Practices
Battery safety is not just about design—it also depends on how batteries are stored, charged, and handled by users. One of the most common causes of thermal runaway is exposure to high temperatures, such as leaving a battery pack in direct sunlight, inside a hot vehicle, or near a heat source. Batteries should always be stored in a cool, well-ventilated area, away from flammable materials.
Charging practices also play a crucial role in preventing battery failures. Users should always use manufacturer-approved chargers that have built-in overcharge protection. Cheap, third-party chargers often lack proper voltage regulation, increasing the risk of overcharging and overheating. Additionally, batteries should never be charged unattended or overnight in an enclosed space, as any abnormal heating during charging could go undetected until it is too late.
Another important precaution is avoiding deep discharge cycles. When lithium-ion cells are discharged below their safe voltage threshold (typically 2.5V per cell), it can cause structural damage inside the battery, making future charging cycles less stable. Maintaining a charge level between 20% and 80% can help prolong battery life while reducing stress on the internal chemistry.
Gas Sensors and Early Warning Systems for Thermal Runaway
Emerging battery safety technologies include gas sensors and thermal runaway detection systems that provide early warning signs before a failure occurs. When a battery cell begins to decompose due to excessive heat, it releases a distinct mix of flammable gases, such as hydrogen and carbon monoxide. Specialized gas sensors can detect these gases in real time and trigger alerts or automatic shutdowns before thermal runaway reaches critical levels.
While gas detection is already used in large-scale energy storage systems and industrial battery applications, it is now being considered for consumer-grade battery packs as well. Future PLEVs may incorporate integrated safety sensors that provide real-time monitoring of battery conditions, allowing users to take action before a catastrophic failure occurs.
The Role of Regulatory Standards and Manufacturer Responsibility
Preventing thermal runaway requires industry-wide collaboration between manufacturers, safety regulators, and policymakers. Governments and safety organizations have introduced stricter battery certification requirements, ensuring that manufacturers adhere to high safety standards before their products reach the market. Regulations such as UL 2271, IEC 62619, and UN 38.3 specify rigorous abuse testing, overcharge protection, and impact resistance criteria to reduce battery-related hazards.
Additionally, battery manufacturers must prioritize quality control and counterfeit prevention. Counterfeit lithium-ion cells, which often lack safety features such as pressure relief valves and thermal shutdown layers, pose a major risk in the consumer market. Ensuring traceability and proper labeling of battery cells can help prevent unsafe batteries from entering circulation.
Conclusion
Thermal runaway is a serious safety concern, but advancements in battery technology, smart management systems, and user awareness can significantly reduce the risks. By improving battery pack design, implementing intelligent BMS monitoring, using safer chemistries, and enforcing best charging practices, manufacturers and consumers can work together to create a safer and more reliable lithium-ion battery ecosystem.
As the demand for electric mobility and renewable energy storage continues to grow, ensuring battery safety must remain a top priority. The future of safe lithium-ion technology will be driven by innovations in thermal management, early detection, and solid-state batteries, ultimately making energy storage solutions more efficient and risk-free for everyday use.
Source: Personal light electric vehicle (PLEV) battery safety research Report


Share:
E-Bike Market in Canada: What’s Next for 2025?
Bridging the Gap: Electric Mobility and the Great Urban-Suburban Divide